
- #Formal charge of carbon in cocl2 trial#
- #Formal charge of carbon in cocl2 plus#
- #Formal charge of carbon in cocl2 free#
two bonding pairs and 16 non- bonding electrons i.e. The OF2 Lewis structure of contains 4 bonding electrons i.e. Thus, the molecular shape of …ġ0.1: Lewis Structures and the Octet Rule - Chemistry LibreTexts Cocl2 lewis structure lone pairs WebIn order to draw the lewis structure of OF2, first of all you have to find the total number of valence electrons present in the OF2 molecule. The three groups of electron pairs are arranged in a trigonal plane. arrangement - AX3 with “3 bonding pairs” & no lone pairs on the central atom). We … altruisme ou egoismeĬocl2 lewis structure lone pairs WebAnswer (1 of 3): The bond angle is 180o. By looking at the OCl 2 Lewis structure shown above, we understand that there are two Chlorine atoms attached to the central Oxygen atom. Scroll to top Follow Us – passive misrepresentation in florida real estate What is the hybridization of the central atom in pf3cl2? Webastor crowne plaza new orleans haunted. Solved Enter the Lewis structure for the molecule of COCl2, - Chegg
#Formal charge of carbon in cocl2 plus#
So let's start out a plus B audition is pretty easy.

It contains 24 …Ĭocl2 Molar Mass - Braineds WebFollowing matrices A and B and perform the following operations A plus B A minus B three a and three a minus to B. This article concludes that it has triagonal planar shape with 111.80 bond angle. WebPhosgene is an organic compound with formula COCl2.
#Formal charge of carbon in cocl2 free#
Nanomaterials Free Full-Text Waste Citrus reticulata Assisted. The electron pairs which are present here are … e commerce home page On central atom there is 2 bonding pairs and 1 lone pairs. WebThe bond angle of Cocl2 Lewis structure is 120. Therefore, In the Lewis structure of I C l 2 −, 3 lone pairs of electrons are around the iodine atom. The Lewis structure of I C l 2 − is shown below.Therefore, the Lewis Structure for the CoCl 2 is represented as follows: CoCl2 … Thus, the Lewis structure of CoCl 2 is an exception to the octet rule.Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a … ecommerce home ios app Name molecule and electron … e commerce help deskĪc B is equal toHint: A" is the complement of A … - SolvedLibĬ2cl2 Lewis Structure - Braineds WebTo determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Recognize the difference between electron and molecular geometry. WebRecognize that molecule geometry is due to repulsions between electron groups. In the Lewis structure of $ICl_2^ -$, how many lone pairs of Literature guides Concept explainers Writing guide Popular.
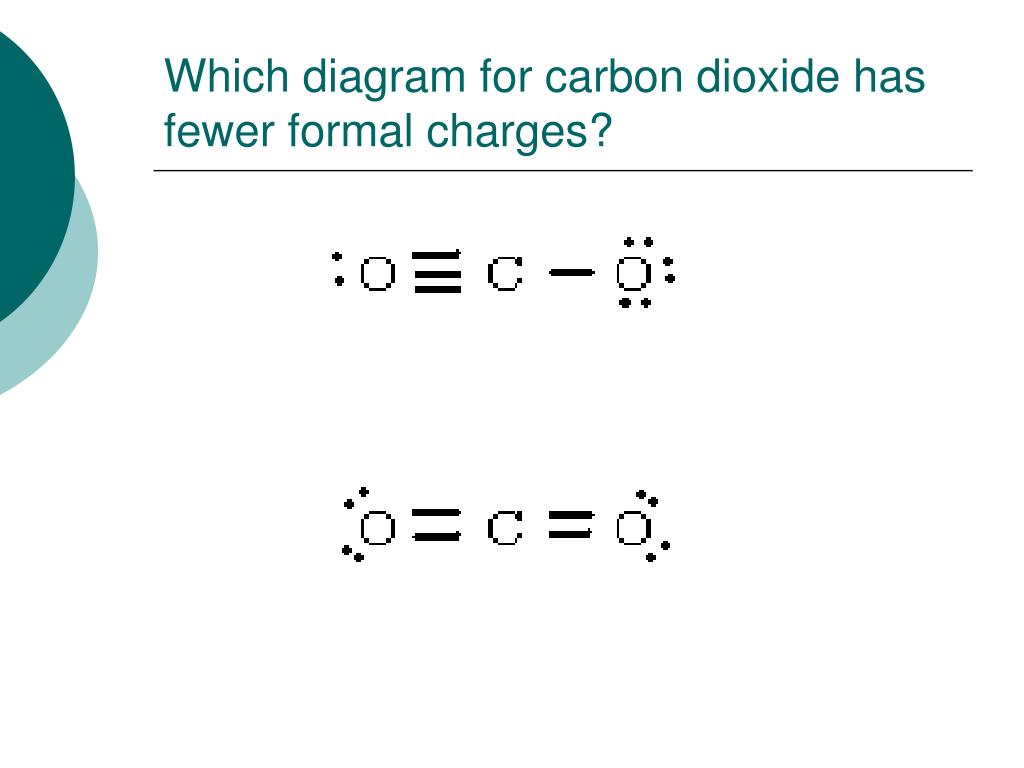
#Formal charge of carbon in cocl2 trial#
Start your trial now! First week only $4.99! arrow_forward. WebSolution for Draw a bond-line structure having the molecular formula +. SOCl2 lewis structure, Molecular geometry, Polar or nonĬaCl2 Lewis Structure, Geometry: 13 Facts You Should Know Draw the molecule by placing atoms on the grid and connecting them with bonds. Steps #1 Draw Sketch #2 Mark Lone Pairs #3 Mark Charges #4 Minimize Charges altruism is unique to servant leadership In the lewis structure of C 2 Cl 2, there is a triple bond between the two carbon atoms, and each carbon is attached with one chlorine atom, and on each chlorine atom, there are three lone pairs.

What are some examples of trigonal planar compounds? - All …Ĭocl2 lewis structure lone pairs WebC 2 Cl 2 (dichloroacetylene) has two carbon atoms and two chlorine atoms. What is the hybridization of the central atom in pf3cl2?ĬOCl2 Lewis Structure, Molecular Geometry, … Cocl2 lewis structure lone pairs …Ĭontrolling Photocatalytic Properties of Eosin Y-Sensitized … WebWhich of the following molecule/species is having maximum number of lone pairs in Lewis-dot structure? A. So, this … ecommerce hobartĬocl2 lewis structure lone pairs Ocl2 Lewis Structure - Braineds There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. Solved Draw the Lewis structure for COCl2, including lone - Chegg WebIt is very easy to draw the Cl 2 lewis structure. Cocl2 Lewis Structure,Characteristics:13 Facts You Should Know


 0 kommentar(er)
0 kommentar(er)
